Meet The New Maestra
By Mytri Vunnam
Teaching Assistant Professor Dr. Jade Fostvedt (Image #1 Source: Jade Fostvedt)
From ferrocene rings to authoring papers Dr. Jade Fostvedt, one of the newest faculty members in the chemistry department shares some details about herself, her time as a graduate student and all she hopes to accomplish at the University of North Carolina at Chapel Hill.
Dr. Fostvedt completed her Bachelor’s in chemistry at the University of South Dakota then went on the University of California, Berkeley for her PhD in synthetic chemistry. Her thesis primarily focused on the early transition metals niobium and tantalum, and she was looking to discover new ways those metals could interact with small molecules, incorporating hydrogen into molecules and more. As she elaborated, this type of research is primarily used as a first step in catalysis. For example, to produce ammonia the Haber-Bosch process is used, which requires remarkably elevated levels of heat and pressure. Using metal-complexes as a catalyst could help turn dinitrogen into ammonia under milder conditions. Ammonia is essential for fertilization of crops and produced at extremely high volumes. Producing ammonia under milder conditions would save vast amounts of energy and make production much more environmentally friendly. Though this is the far reaching application of Dr. Fostvedt’s research, it is the first fundamental step in the process.
Surprisingly by the end of graduate school Dr. Fostvedt says she enjoyed writing papers. While at first Dr. Fostvedt was incredibly stressed out by them, eventually she grew to like writing papers, because it was a chance to present her work and, “show how you solved all these little mysteries every day to make this bigger project.”
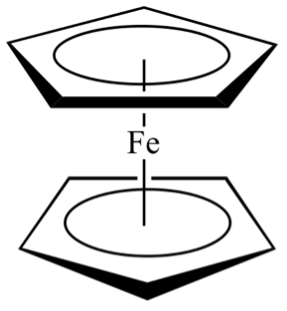
Dr. Fostvedt now teaches General Chemistry and Inorganic Chemistry at UNC. She is quoted in saying that she loves teaching, and that the students here take class seriously but also have a work-life balance and strong community values. She also says everyone has been extremely nice. She states that her main goal, by the end of the semester, is to modify the inorganic curriculum so it is more problem-solving based rather than lecture based, and to work in some live chemistry demonstrations in class, like with color changing ferrocene. Ferrocene is an orgo-metallic compound, with two carbon rings bound to a central iron atom. The substance is originally yellow but when reacted with acetylene it turns red and when reacted with iron (III) chloride, it turns a dark blue. It is a great first fundamental lab to demonstrate some inorganic chemistry concepts.
A molecular drawing of a ferrocene molecule
The physical substance, which is naturally yellow-orange in color.
Source: Science Direct - https://www.sciencedirect.com/topics/chemistry/ferrocene
Dr. Fostvedt’s ultimate goal is to establish UNC’s first ever Inorganic Chemistry Teaching Lab. Inorganic Chemistry is famously the only chemistry class that does not have a lab attached to it. General Chemistry, Organic Chemistry, and Analytical Chemistry all have labs attached to the lecture, and Dr. Fostvedt’s goal is to bring Inorganic Chemistry to the same level. As she describes, inorganic labs have lot of airless equipment, most importantly including a Schlenk line and a glove box. These are both necessary for manipulating inorganic compounds without touching the air as inorganic compounds are usually highly reactive with water and oxygen. Students would have an opportunity to make or modify molecular compounds and then characterize them. She elaborates that characterizing compounds requires an Nuclear Magnetic Resonance facility which uses state-of-the-art spectrometers to determine the structures of small molecules. UNC has the resources to provide such processes to even teaching labs due to being a well-funded R1 institution.
A Schlenk line, used to manipulate inorganic compounds without exposing them to the air
(Image #3 Source: The Schlenk Line Survival Guide - https://schlenklinesurvivalguide.com/gallery/)
In her own words, a lab is particularly important because “chemists try to represent chemistry in so many ways, like symbolic representations with math or chemistry formulas. Or pictorial representation like molecular orbitals and Lewis dots.” However, we are missing the third representation which is how it looks in real life. The lab is the third representation of the topic as “it helps students make ties to what they see all around them and helps symbolic and pictorial representations fit into the framework of knowledge that already in their head.”
There is still so much to expand upon and new things to teach in the chemistry program here, and Dr. Fostvedt is taking the next step in that process. Students at UNC are excited to welcome Dr. Fostvedt’s experience and endeavors here, and hope she can help further develop the chemistry department.
References
1. Interview with Dr. Jade Fostvedt, Teaching Assistant Professor, 9/14/2022
2. Fostvedt, J. (2022), Design of Niobium and Tantalum Systems for Small Molecule Activation, [Doctoral Dissertation, University of California at Berkeley]. eScholarship University of California