3D Printing A More Sustainable Future
By Anna Vu
Imagine if you could turn an idea in your head into a physical product in a blink of an eye. You do not have to imagine with 3D printing. Although 3D printing may seem like a recent development, 3D printing has existed for about four decades. It first originated in Japan in the early 1980s by a man named Hideo Kodama, who made a rapid prototyping machine that stacked polymers layer by layer (3). Polymers are materials made up of multiple monomers, which are small building blocks or molecules. Other forms of 3D printing were created, such as stereolithography (SLA) made by Charles Hull and selective laser sintering (SLS) made by Carl Deckard (3).
The National Academy of Sciences defines 3D printing as “build[ing] three-dimensional objects based on instructions in computer files.” A person designs the product from a computer program or software, sends the data to the printer, and prints out the product using materials like resin, plastic, metal, or cells (2). The primary material used in 3D printing, however, is plastic, which is not sustainable or good for the environment. Dr. Mia Borden, a postdoctoral research associate in the Leibfarth Group and Chemistry Department at the University of North Carolina at Chapel Hill, is working on developing new materials that can be used in 3D printing, particularly those that are biodegradable and biocompatible.
Dr. Borden completed her doctoral studies in fundamental catalysis and looked at ways to design catalysts as a way to make sustainable materials. Catalysis is the process of changing the rate of a chemical reaction by adding a catalyst, which is not used up in the reaction and can be reused in other reactions. Through catalysis designs, bonds in compounds can be created or broken to then “diversify the properties of materials you can get out of a 3D printing setup” (1). She then joined the Leibfarth group in August 2020 to apply her knowledge in the microscopic field, learn more about polymer science, and build up a well-rounded skill set to become a faculty member for an interdisciplinary studies program in the future. The Leibfarth group is known for their innovative discoveries in polymer science and has three main areas of research: upcycling, steroid chemistry, and polymer networks. Upcycling refers to taking commodity materials that are hard to biodegrade, like plastic, and using chemistry to turn them into different products and make them have new functionality. Steroid chemistry is all about changing the properties of materials, such as using different monomers to make a material more crystalline, solid, and therefore, more durable. Polymer networks are the area of research that Dr. Borden works in, which is developing new methods for 3D printing (1).
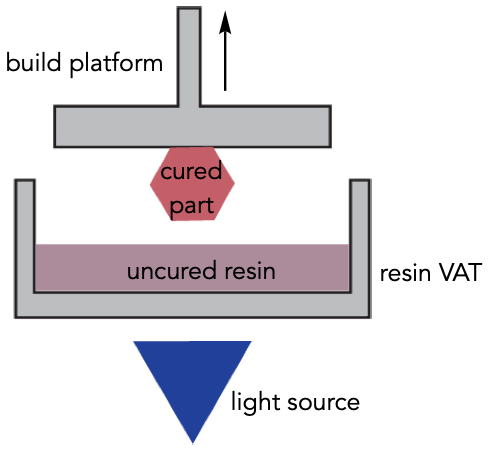
The main machinery used in the research is photopolymerization 3D printing technology, a technique that uses light to cure or solidify the resin, which is a liquid material used to build the product. This commercial, light-based printer can print out materials faster and at a higher resolution than typical 3D printers. The material produced is then tested through a multitude of different experiments. One experiment involves a nitrogen-regulated glove box, which is a box that represents a nitrogen atmosphere so that any processes within that box would not be contaminated from the outside environment. This is used to monitor how fundamental reactions work in the absence of water or air. Within this box, two techniques are used: nuclear magnetic resonance (NMR) and size-exclusion chromatography (SEC). NMR measures the quantity of a polymer made and SEC is a separation technique that separates smaller molecules from bigger molecules so that the size of the polymer can be tested. Dr. Borden also uses tensile testing in her research, which measures the force of the material. This relates to how much the material can be stretched, how strong the material is, and the exact force needed to break the material. She also determines the thermal properties of a material by placing a sample of the material in the oven on a scale, and measuring the weight of the material as it burns up. The method reveals the mechanical properties of the material and how thermally stable it is (usually from 200-400 degrees), which is useful if you want a heat-resistant object. All of these experiments work together to determine the overarching properties of a material that is produced from a 3D printer, which is crucial to see how a material can be transformed into a usable product in real-world applications (1).
Dr. Borden is currently working on using catalytic chemistry in the 3D printing setup, along with implementing bio-derived monomers in the resin, which is made out of mostly plastics, so that the materials are more sustainable. She is also researching the degradation of materials to see if any of them can turn into possible biodevices or change their degradation times to be faster. Her work in the lab is quite young at only a year old, so there are still many avenues to discover. She plans to publish her research in the near future that contains all of the complete and necessary data, including the setbacks in her research, which is important so that scientists can replicate and use the chemistry as efficiently as possible.
With all of these scientific developments in 3D printing, it may seem exclusive and inaccessible. However, there are applications in everyday life with 3D printing. Companies have been using 3D printing to make products starting from the 1990s, and in 2006, the first 3D printer was commercially made available to the public (3). Dr. Borden says that “3D printing is good for anything with customization” (1). For example, the dental industry relies on 3D printing to make models of teeth and the medical industry uses 3D printing for biomedical devices. Humans are made of all shapes and sizes, including their feet, so when making insoles in your shoes, 3D printing is a useful way to customize insoles that fit and can have adjustable levels of impact. Before 3D printing, companies would use molding models to produce prototypes, which would take more effort and time to make because a different mold would have to be made for every design. With 3D printing, prototypes can be made much quicker, some as short as under an hour.
Dr. Borden’s research unlocks the many possibilities of 3D printing. It is an exciting way to develop biodegradable materials ranging from the insoles in your shoes to entire buildings. In the next couple of years, we could be living in a completely different world made up of sustainable materials, all thanks to polymer chemistry and 3D printing.
References
Interview with Meredith (Mia) Borden, Ph.D. 2/23/22.
What is 3D printing? https://thesciencebehindit.org/what-is-3d-printing/. (accessed February 25th, 2022).
When Was 3D Printing Invented? The History of 3D Printing https://www.bcn3d.com/the-history-of-3d-printing-when-was-3d-printing-invented/. (accessed February 25th, 2022).